The free energy of the system decreases. An exergonic reaction will decrease the free energy of a system while an endergonic reaction will increase it.

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
Electrons tend to have more potential energy when _ in.

. The converse is an endothermic reaction. In a system energy can do work and energy can be changed to other forms such as heat sound light etc. The reaction releases free energy.
Exergonic reactions are the spontaneous reactions that give out energy in the form of heat or vapour. In chemical thermodynamics an exergonic reaction is a chemical reaction where the change in the free energy is negative there is a net release of free energy. The energy required for a reaction to proceed by breaking bonds.
The chemical bonds formed from the reaction are stronger than those that were broken in the reactants. The catalysts needed to raise a reactions rate. The free energy is constant and doesnt change whether if there is a catalysis enzyme.
Reactions that have a negative G release free energy and are called exergonic reactions. For the toolbar press ALTF10 PC or ALTFNF10 Mac. For processes that take place in a closed system at constant pressure and temperature the Gibbs free energy.
Which of the following correctly describe an exergonic reaction. Exergonic reactions release energy to the surroundings. A reaction in which energy is lost the reaction goes from a high energy state to a low energy state is considered an exergonic reaction.
First law of thermodynamics. Both of these reactions relate to the enthalpy change of the process. The key difference between endergonic and exergonic is that endergonic reactions are non-spontaneous and unfavourable whereas exergonic reactions are spontaneous and favourable.
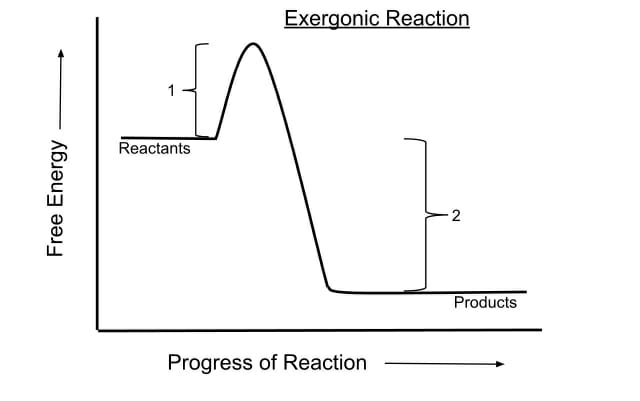
Submitted by fiddleman on Wed 03022011 - 1604. You must expend more energy than you receive. The reactants are higher in energy than the products because energy must be released as the products are being formed.
The exergonic reaction releases energy whereas the endergonic reaction consumes energy. Which word describes the energy in an exergonic reaction. The change in the standard Gibbs Free Energy G of an exergonic reaction.
As the chemical reaction proceeds the reactants are being converted into products. They must be supported by empirical data. A well-tested explanation of natural events.
Endergonic and exergonic reactions. Energy cannot be created or destroyed. A reaction where energy is gained requires some form of energy input and is considered nonspontaneous.
In chemistry terms exergonic reactions are reactions where the change in free energy is negative. Applying the following equation. Endergonic reactions are also known as nonspontaneous reactions or unfavourable reactions.
In such a case I believe that the activation energy for the forward exergenic reaction would be less than for the reverse reaction. The energy required for a reaction to proceed by breaking bonds d. Energy is the capacity to do work.
Reduction is generally defined as the gain of electrons. The transition state differs because in an exergonic reaction without a catalysis enzyme it takes longer for the transition. B I U Ꭶ Paragraph Arial 10pt А м 1 жова P O WORDS POWERED BY TINY QUESTION 3 1 points Save Answer Does the following describe an exergonic or endergonic reaction.
ΔS is the entropy change and T is the temperature. An exothermic reaction is one whereby energy is released from the system into the environment usually in the form of heat or light. Alternatively endergonic reactions may also be referred to as unfavorable reactions or involuntary reactions.
An exergonic reaction is a kind of spontaneous reaction where theres release of free here free energy is negative under zero. Free energy measures the total amount of energy available in a system. An exergonic reaction may be called a spontaneous reaction or a favorable reaction.
Not completely familiar with the term exergenic but I believe that they are reactions with a negative change in free energy. A type of endergonic reaction c. On the other hand endergonic reactions would be the reactions where energy enters the machine the disposable energy heres positive more than.
Since the reactants are losing. Because this type of reaction releases energy rather than consuming it it can occur spontaneously without being forced by outside factors. Negative changes mean that energy has been.
ΔG ΔH TΔS. Some examples are Oxidation of glucose Oxidation of NADH to NAD. In an exergonic reaction what is the DeltaG.
EXergonic means energy is EXiting the system A negative G means that the reactants or initial state have more free energy than the products or final state. What must be true of theories before they are accepted by the scientific community. Where ΔH is the enthalpy of the reaction that is the total energy released or absorbed.
The reaction necessitates further energy than you receive. DeltaG is negative which means that the reaction is spontaneous in the forward direction. Which best describes the energy of activation.
The TΔS factor is the energy loss not used in the expansion or arrangement of the molecules in a phase solid liquid or gas. Oxidation is usually considered exergonic reaction as it involves release of energy. According to the graph above the activation energy for the exergonic reactions without a catalysis enzyme is much higher opposed to the reaction with a catalysis enzyme.
This indicates a spontaneous reaction if the system is closed and initial and final temperatures are the same. The products have lower Gibbs free energy than the reactants. A type of exergonic reaction b.
Exergonic reactions lose energy during the reaction and are spontaneous. The free energy change of the reaction is greater than zero. An exergonic reaction is one whereby energy is also released from the system into the environment.

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy
Exergonic Reaction Definition Examples And Quiz Biology Dictionary

Exergonic Example Chemical Reaction Process What Is An Exergonic Reaction Video Lesson Transcript Study Com
0 Comments